Go ssm air is primarily a mixture of nitrogen n molecular mass 28 0 u and oxygen o molecular mass 32 0 u. In order to maintain the outlet pressure the feed flow rate has to.
.gif)
Compute answers using wolfram s breakthrough technology knowledgebase relied on by millions of students professionals.
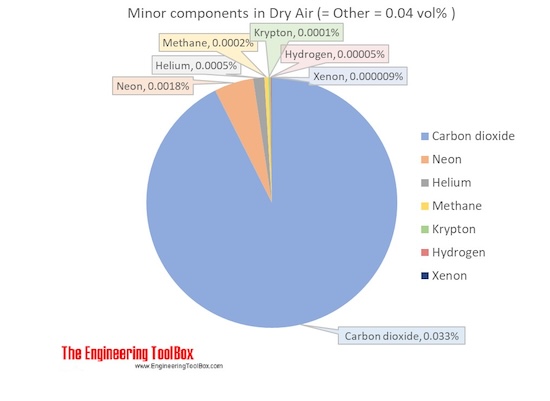
Molecular mass of air. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen. Oxygen has a molar mass of 15 9994 g mol and nitrogen has a molar mass of 14 0067 g mol. The molecular weight of air is approximately 28 97 g mol.
The molecular mass is estimated using the weights of all of the elements contained in air. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen. Oxygen has a molar mass of 15 9994 g mol and nitrogen has a molar mass of 14 0067 g mol.
Dry air has a molar mass of 0 029 kg. Pollution in the air is not only a threat to the environment but to human lives. Compute answers using wolfram s breakthrough technology knowledgebase relied on by millions of students professionals.
Using 1 25 atm of propane in a 150 ml. In order to maintain the outlet pressure the feed flow rate has to. Displaystyle m rm d is the molar mass of dry air.
The specific gas constant for dry air is 287 058 j kg k in si units and 53 35 ft lbf lb r in united states customary and imperial units. This quantity may vary slightly depending on the molecular composition of air at a particular location. Assuming air has an average molecular mass of 27 3 grams mole calculate the mass of air in grams in 1 0 liter of air at 1 0 atmosphere of pressure at 25 degrees c which is 298 k.
The molecular mass m is the mass of a given molecule. It is usually measured in daltons da or u different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. This is distinct but related to the molar mass which is a measure of the average molecular mass of all the molecules in a sample and is usually the more.
Go ssm air is primarily a mixture of nitrogen n molecular mass 28 0 u and oxygen o molecular mass 32 0 u. Assume that each be haves.