Oxygen has a molar mass of 15 9994 g mol and nitrogen has a molar mass of 14 0067 g mol. According to avogadro s law the total number of molecules remains the same in the container under the same conditions volume pressure temperature.

Atomic weight is not the final determinant.
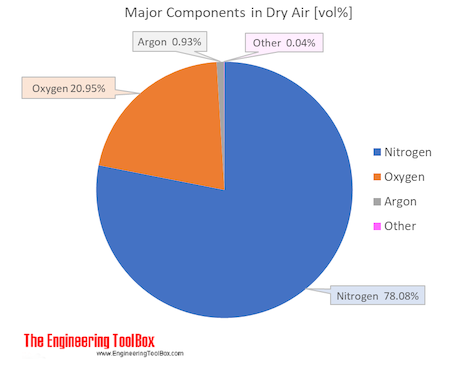
Atomic weight of air. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen. Oxygen has a molar mass of 15 9994 g mol and nitrogen has a molar mass of 14 0067 g mol. The molecular weight of air is approximately 28 97 g mol.
The molecular mass is estimated using the weights of all of the elements contained in air. Air is mostly made of oxygen and nitrogen. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen.
Oxygen has a molar mass of 15 9994 g mol and nitrogen has a molar mass of 14 0067 g mol. Since both of these elements are diatomic in air o2 and n2 the molar mass of oxygen gas is 32 g mol and the molar mass of nitrogen. World wide web version of atomic weight data originally prepared by g.
Moss from a file provided by d. Previous values may be consulted from the 1993 table the 1995 table the 1997 table the 1999 table the 2001 table the 2005 table the 2007 table the 2009 table the 2011 table the 2013 table or the 2015 table. An isotope is one of two or more species of atoms of the same chemical element that have different atomic mass numbers protons neutrons.
The atomic weight of helium is 4 002602 the average that reflects the typical ratio of natural abundances of its isotopes. Atomic weight is measured in atomic mass units amu also called daltons. 1 molecules of argon ar ar has one atom with atomic weight 39 8 u.
Note that every molecule listed is heavier that or equal to 18 u. Now let s add some water vapor molecules to the gas with the total atomic weight of 18 u h o two atoms of hydrogen 1 u and one oxygen 16 u. According to avogadro s law the total number of molecules remains the same in the container under the same conditions volume pressure temperature.
Argon is a chemical element with the symbol ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in the earth s atmosphere at 0 934 9340 ppmv it is more than twice as abundant as water vapor which averages about 4000 ppmv but varies greatly 23 times as abundant as carbon dioxide 400 ppmv and more than 500 times as.
Atomic weight is not the final determinant. However as the term heavier than air is usually taken to mean more dense if nitrogen has a higher density than oxygen it should sink in the presence of oxygen regardless of its atomic or molecular mass.